Placebo & Bioplausability Bias







WHY THEORIES ARE NOT CURES
—-
The Placebo Effect
The placebo effect is a very well-documented phenomenon in medicine. In short: if you give someone a drug* and they believe it will help their symptoms they will report and even experience feeling better. It is the power of suggestion and of confirmation bias combined.
The placebo effect is most powerful in subjective symptoms like mood, pain, anxiety, brain fog, and fatigue. But it applies to almost all conditions.
Because MECFS and Long Covid all have many subjective symptoms (alongside objective ones) it is especially important to control for the placebo effect when studying these diseases.
*This post will primarily use the terms drug and medication but this also applies to herbs, supplements, and non-drug therapies
—-
Evidence For Treatments
The placebo effect is the reason that for medications to be considered effective they must pass "double blind trials" and/or "placebo controlled trials."
There are a few key elements to evaluate when determining the quality of a study
1.) Placebo control - If a trial does not control for placebo bias it will often produce positive effects in diseases with high placebo effect like MECFS and long covid regardless of whether the treatment is effective beyond a placebo.
2.) Sample size - If a trial is too small it is possible that positive results are simply a matter of luck, or that the statistics can be manipulated to produce some positive results regardless of actual outcome. Look for trials of at least 100 patients and preferably 1,000.
3.) Selection bias - If the way patients are chosen for a trial is biased this can skew the results. For example, not requiring post exertional malaise will likely lead to patients with MECFS being included in the trial. Choosing patients based on attendence of an in-person clinic will exclude severe patients.
4.) Analysis bias - While the data from a trial is a key element of the quality of a study, it is not all that determines it. The authors must then analyze the data to produce results and a conclusion. If this analysis is biased it can deeply skew the conclusions of a study.
—-
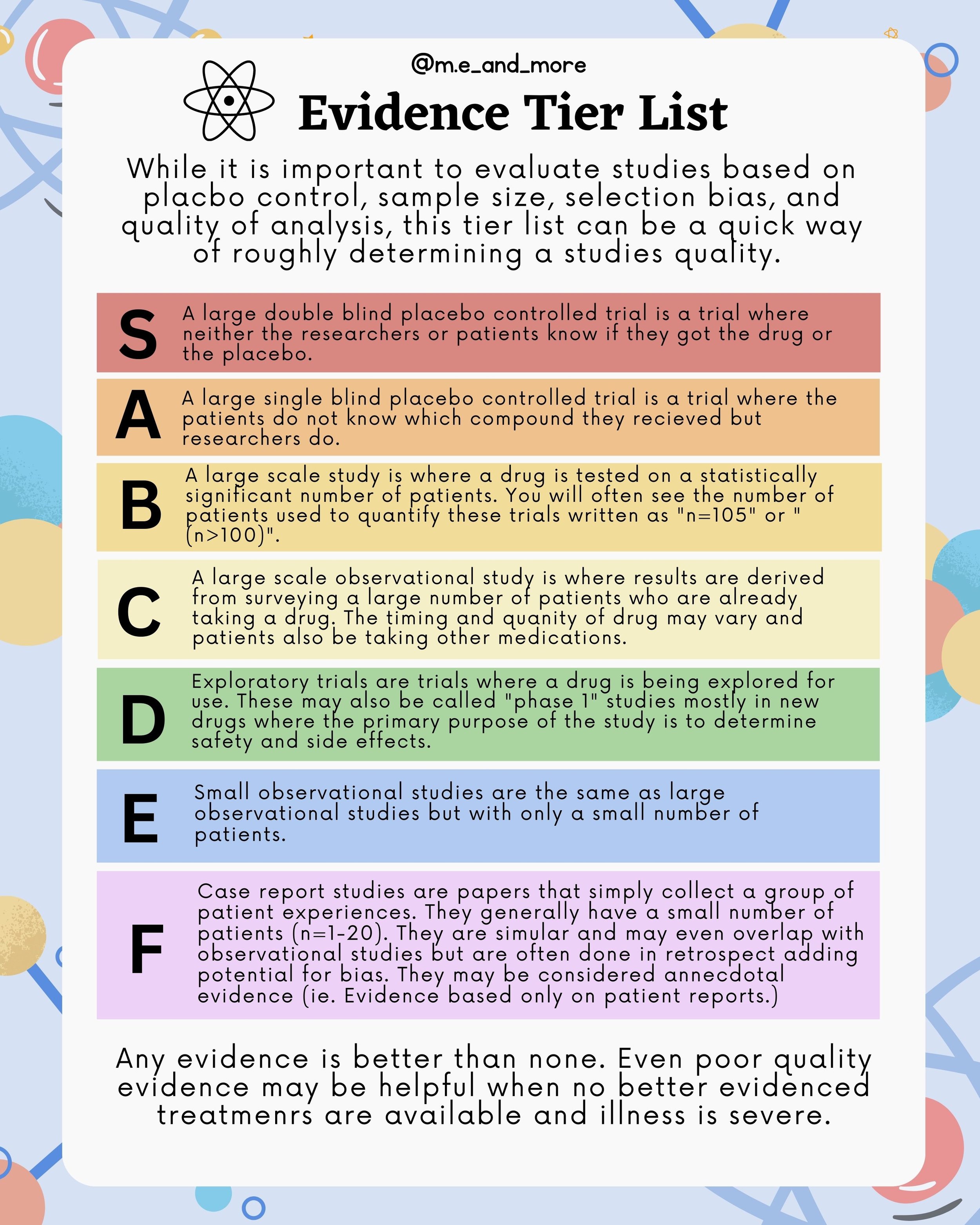
Evidence Tier List
While it is important to evaluate studies based on placbo control, sample size, selection bias, and quality of analysis, this tier list can be a quick way of roughly determining a studies quality.
S - A large double blind placebo controlled trial is a trial where neither the researchers or patients know if they got the drug or the placebo.
A - A large single blind placebo controlled trial is a trial where the patients do not know which compound they recieved but researchers do.
B- A large scale study is where a drug is tested on a statistically significant number of patients. You will often see the number of patients used to quantify these trials written as "n=105" or "(n>100)".
C - A large scale observational study is where results are derived from surveying a large number of patients who are already taking a drug. The timing and quanity of drug may vary and patients also be taking other medications.
D - Exploratory trials are trials where a drug is being explored for use. These may also be called "phase 1" studies mostly in new drugs where the primary purpose of the study is to determine safety and side effects.
E - Small observational studies are the same as large observational studies but with only a small number of patients.
F - Case report studies are papers that simply collect a group of patient experiences. They generally have a small number of patients (n=1-20). They are simular and may even overlap with observational studies but are often done in retrospect adding potential for bias. They may be considered annecdotal evidence (ie. Evidence based only on patient reports.)
Any evidence is better than none. Even poor quality evidence may be helpful when no better evidenced treatmenrs are available and illness is severe.
—-
Bioplausability
If something is bioplausable it means that there is a theory based in our knowledge about chemical compounds and their interactions with the body that would explain a medication works.
Bioplausability is the basis of most medical treatment trials. If there is not a mechanism by which a drug could work then it is unlikely to be tried on an illness in the first place.
When a patient is gravely ill it is reasonable to trial a medication that has a bioplausable mechanism for helping their symptoms even if that medication has not been proven effective.
—-
Lots Of Medicine Works But Is Ineffective
Over 90% of drug trials fail. That means they do not work better than placebo when trialed in large single or double-blinded studies.
But many of these drugs do "work." That is to say that the patients in the studies who were given the drug report positive outcomes or feeling better. They just don't feel any better than the placebo group.
This is why so many small-scale non-placebo controlled studies produce positive results. Without a placebo group, anything that doesn't make the patients feel worse is likely to make them feel better simply because of the placebo effect.
—-
The Power of Bioplausability in the MECFS & Long Covid Community
Because MECFS and Long Covid have no proven treatments, the only way for practitioners to support their use of various drugs is through arguments of bioplausability.
These arguments are extremely convincing. In fact, they are often more convincing than the single-line explanation "this worked in a placebo-controlled trial."
Most patients with MECFS know that water doesn't have memory. So they won't believe a homeopathic medicine is going to work. But they likely will believe that they have a latent viral infection that can be treated with Valtrex. That means that whether Valtrex works or not, it will have a stronger placebo effect.
It also means that simply by explaining bioplausable mechanisms for a drug, doctors and bloggers are in an essence marketing it. Because people are more convinced to try something bioplausable.
—-
Humans and Stories
Humans love a story-based explanation. We want things to have a clear cause and effect we can understand.
For example, even though the chemical imbalance theory of depression has been shown not to be accurate, many psychiatrists still use it to explain why antidepressants work to their patients. It is much more convincing than "SSRIs work better than placebo in randomized controlled trials."
The theory being incorrect doesn't make the medication ineffective. But clinicians and patients alike are drawn more to the story explanation that there is a deficit of serotonin and this drug corrects it, than to the real explanation of "we don't know why increasing serotonin works but it is an effective treatment for major depressive disorder."
—-
How Reliance On Stories Harms People With MECFS
For people with ME, the propensity of doctors to defect towards reasonable story based explanations has lead to the proliferation of the biopsychosocial model and variations on it. This has done irreperable harm to our community.
Graded excercise therapy (GET) does not work. It has never worked. But it has an extremely persuasive story: "People with ME got sick with a virus and became deconditioned leading to long term illness. If you recondition them with GET they will get better."
Even as evidence based guidelines have changed against GET, doctors continue to prescribe it because the story sticks in their head better than objective evidence.
—-
The Takeaway
-Theories of causes of MECFS and Long Covid are essential to developing new treatments
-Bioplausability is a necessary first step in treatment research
-The placebo effect means that treatments can "work" without being effective
-Most bioplausable treatments are not effective
-A bioplausable explanation is not enough to recommend a treatment as effective
-We must be cautious not to jump to conclusions about treatment efficacy based only on non-placebo controlled trials and bioplausable arguments
-Its important to evaluate studies of treatments based on placebo control, sample size, selection bias, and analysis bias
This post is inspired by an excellent hour long video "Why Do People Keep Falling For Things That Don't Work" by Med Life Crisis. For more excellent evidence-based content please check out their channel.
—-
Myalgic Encephalomyelitis has no cure. It has no FDA-approved treatments. For pwME that is a very hard truth to swallow.
⠀
The term evidence Based Medicine is not clear-cut. What evidence counts? What does it mean for something to be effective vs an effective treatment?
⠀
This post aims to illustrate how we can judge the evidence around experimental Treatments as well as the danger of ignoring the placebo Effect and #mechanisticBias or bioplausability Bias.
⠀
People with MECFS desperately want to get better. Pacing the best evidenced "treatment" for ME / CFS means rationing your life. Putting everything on hold.
⠀
Our choices are to wait in pain and confinement for underfunded and desperately needed research for MECFS or to try expensive rarely funded treatments based on evidence ranging from not quite there yet to "my cousin's friends sister said."
⠀
The quality of life in ME particularly severe ME is so low that it is reasonable to trial medications that are unproven. But it is essential that we learn to choose medications based on evidence.
⠀
Ampligen, an immunotherapy only available in Argentina, is the only drug approved anywhere in the world for MECFS. It has had placebo-controlled studies. Yet it is barely discussed.
⠀
LDN, LDA, Nimodipine, Mestinon, even BC007 these drugs are promising yes, but none have had large scale placebo trials. We desperately need to know if these drugs work. Not just keep relying on observational studies in a disease with known extreme placebo effect.
⠀
Then there is the question of using drugs whose efficacy is split, like IVIG and Humira where some patients respond well and others poorly.
⠀
Anyone who tells you they know what causes this disease is lying. This patient population is held together by Post Exertional Malaise or PEM. But we are also extremely heterogeneous. We don't know if there even is a single cause of this disease.
⠀
There is no cure for MECFS. There are no FDA approved treatments for MECFS. To cure MECFS to solve MECFS we need research. 40 years ago would be nice. I'll settle for now.
⠀
Please support @openmedf and @solveMECFS and give us hope.